When a cement slurry is placed across a permeable formation under pressure, a filtration process occurs. The aqueous phase of the slurry escapes into the formation, leaving the cement particles behind. Such a process is commonly known as fluid loss ( one of the oil well cement properties) and it is put under control in the cement by certain agents. As you are here, we think you might be interested in Cementing Additives Article.
If cement fluid loss is not controlled, several serious consequences may result that can lead to cement-job failure. As the volume of the aqueous phase decreases, the slurry density increases; as a result, the slurry performance diverges from the original cement design. If sufficient fluid is lost to the formation, the slurry becomes unpumpable.
The API fluid-loss rate of a neat cement slurry generally exceeds 1,500 mL/30 min. An API fluid-loss rate less than 50 mL/30 min is often required to maintain adequate slurry performance. To accomplish such a reduction in the fluid-loss rate, materials known as fluid-loss control agents are included in the slurry design.
Cement Fluid Loss Control Agents Mechanism
The exact mechanisms by which fluid-loss control agents operate are not completely understood; however, several processes are known to occur. Once cement fluid loss commences across a formation, a filter cake of cement solids is deposited on the formation surface. Fluid-loss agents decrease the filtration rate by reducing the filter cake permeability, increasing the viscosity of the aqueous phase, or both. Two principal classes of fluid-loss additives exist:
- Finely divided particulate materials
- Water-soluble polymers.
Cement Fluid Loss Control Agents – Particulate materials
The first fluid-loss control agent used for cement slurries was bentonite. Because of the small size of its platelets, bentonite can enter the filter cake and lodge between the cement particles, decreasing the permeability of the filter cake. In addition to bentonite, particulate systems such as carbonate powder, carbon black, micro silica, asphaltenes, and thermoplastic resins are used to control cement fluid loss.
Latex cements
The Latex cements demonstrate excellent fluid-loss control. Latexes are emulsion polymers, usually supplied as milky suspensions of very small spherical polymer particles (generally between 30 and 200 nm in diameter). Most latex dispersions contain about 45% solids. Like bentonite, such small particles physically plug small pores in the cement filtercake.
Latex cement demonstrates excellent fluid-loss control. Latexes are emulsion polymers, usually supplied as milky suspensions of very small spherical polymer particles (generally between 30 and 200 nm in diameter). Most latex dispersions contain about 45% solids. Like bentonite, such small particles physically plug small pores in the cement filter cake.
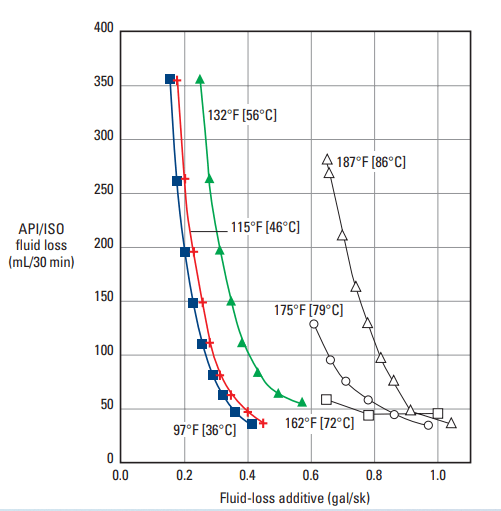
The most common latexes for well cements are those of vinylidene chloride, polyvinyl acetate, and styrene-butadiene. The first two materials are limited to temperatures below 122°F [50°C]. Styrene-butadiene latex has been applied at temperatures up to 375°F [191°C]. Figure 1 is a plot of fluid-loss rate versus styrene-butadiene-latex concentration for various cement slurries.
![Fluid-loss behavior of latex-modified cement slurries at 185°F [85°C].](https://www.drillingmanual.com/wp-content/uploads/2021/04/image-76.png)
Crosslinked PVA
A newer particulate cement fluid loss additive, based on crosslinked polyvinyl alcohol (PVA) microgels, was introduced by Audebert et al. in 1997. It provides excellent fluid-loss control at temperatures up to 250°F [121°C] (Fig.2). This additive does not retard cement hydration and is compatible with cement accelerators. Thus, it is particularly suitable for low-temperature applications, for which short waiting-on-cement times are difficult to obtain. Crosslinked PVA can also be used in combination with polyvinylpyrrolidone (Moulin, 2001). Cement slurries prepared with this additive combination show excellent gas-tight properties. Such additives can be used in combination with microcement and other chemicals for squeeze cementing during which a high degree of fluid-loss control is often required (BarletGouédard et al.).
The fluid-loss behavior of cement slurries containing particulate fluid-loss additives is different from that observed when employing water-soluble polymers. The leakoff is not a function of the square root of time. Instead, an initial filtrate spurt occurs that corresponds to the formation of a thin filtercake. Then the fluid-loss rate is very low, owing to the low filtercake permeability.
Cement Fluid Loss Control Agents – Water soluble Polymers
Water-soluble polymers received much attention as fluid-loss control agents in the early 1940s, when they were first used in drilling fluids. Today, such materials are used extensively as fluid-loss control agents for well cement slurries. In general terms, they operate by simultaneously increasing the viscosity of the aqueous phase and decreasing the filter cake permeability.
Water-soluble Polymers
Polymer Solution Viscosity
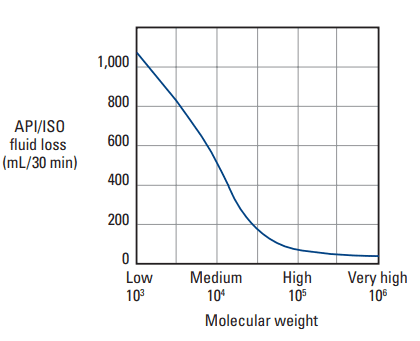
The viscosity of a polymer solution is dependent upon the concentration and the MW. For example, as seen in Fig. 3, a 2 wt% solution of low-molecular-weight HEC may have a viscosity of 500 cp, but the viscosity of an equally concentrated solution of high-molecular-weight HEC can be as high as 50,000 cp. Such a high viscosity would certainly decrease the filtration rate; however, this strategy alone cannot be relied upon to provide fluid-loss control, because slurry mixing would be impossible.
![Concentration and molecular-weight effect on viscosity of aqueous solutions of HEC at 77°F [25°C].](https://www.drillingmanual.com/wp-content/uploads/2021/04/image-78.png)
Filter Cake Permeability
Reduction of filter cake permeability is the more important parameter for fluid-loss control. When a slurry contains sufficient fluid-loss control agent to provide an API/ISO fluid-loss rate of 25 mL/30 min, the resulting filter cake is approximately 1,000 times less permeable than that obtained with a neat slurry. In this case, the interstitial water viscosity increases, at most, five times (Table 1).
![The efficiency of Different Polymers in Decreasing Cement Filtercake Permeability and Increasing Filtrate Viscosity at 80°F [25°C]](https://www.drillingmanual.com/wp-content/uploads/2021/04/image-79.png)
The size of the pores in the cement filter cake can be evaluated by mercury porosimetry. The typical size distribution is shown in Fig. 4, which shows the median diameter to be 1 μm. The typical radius of gyration of a polymer molecule is less than 1,000 Å [0.1 μm]; therefore, only clusters of molecules would be sufficiently large to obstruct a pore in the filter cake. Water-soluble polymers can form weakly bonded colloidal aggregates in a solution that are sufficiently stable to become wedged in the filter cake constrictions (Christian et al., 1976). Such polymers may also adsorb onto the cement grain surfaces and thus reduce the pore size. More likely, a superposition of these two phenomena, adsorption plus aggregation, is the true mechanism of action of polymeric fluid-loss agents.
![Pore diameters of two Class G cement filtercakes (15.8 lbm/gal [1,900 kg/m 3 ], with 0.5% PNS BWOC, no fluid-loss additive).](https://www.drillingmanual.com/wp-content/uploads/2021/04/image-80.png)
Dispersion Of Water Soluble Polymers
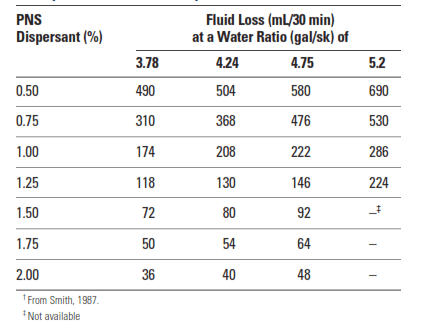
Cement slurries containing water-soluble polymers must be well dispersed to obtain optimal fluid-loss control. Sulfonated aromatic polymers or salts are almost always added with these materials. Cement Dispersants improve the packing of cement grains (and perhaps the polymer aggregates) in the filter cake. Thus, as shown in Table 2, dispersants reduce the permeability of the cement filter cake and can provide some degree of fluid-loss control on their own. However, one must bear in mind that slurry overdispersion and sedimentation may artificially improve the results of the API/ISO fluid-loss test.
Unlike particulate fluid-loss additives, water-soluble polymers do not promote the formation of a thin and impermeable cement filter cake. Instead, they simply reduce the rate at which the filter cake thickens. This process continues until the slurry dehydrates, leaving a thick filter cake.
Several classes of water-soluble polymers are used as fluid-loss control agents. The chemical properties and performance of each are discussed separately in the following sections.
Water-soluble Polymers Cement Fluid Loss Control Agents
Natural Polymers – Cellulose derivatives
The first fluid-loss additive based on a water-soluble polymer was a protein (i.e., a polypeptide) extracted from soybeans. Shortly thereafter, ethylene diamine carboxymethylcellulose and other cellulose derivatives were introduced. In the late s, CMHEC was introduced as a fluid-loss additive for cement slurries. The basic unit structure of CMHEC is shown in Fig. 5.
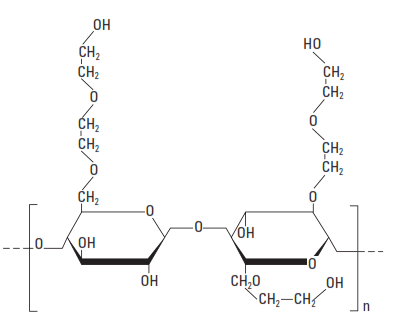
More recently, the performance of CMHEC has been improved by adjusting the DS from 0.1 to 0.7 (carboxymethyl) and the mole ratio of ethylene oxide to anhydroglucose (by MS) from about 0.7 to about 2.5. According to Chatterji et al. (1984) the performance of CMHEC in salt slurries can be improved by adding a hydroxycarboxy acid such as tartaric acid.
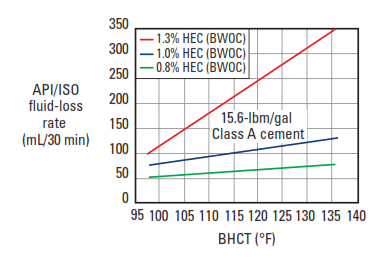
The most common cellulosic fluid-loss control agent is HEC, with a DS range between 0.25 and 2.5 (Hook, 1969). The basic structural unit is shown in Fig. 5. Various MWs of the polymer are used, depending upon the density of the cement slurry. For normal-density slurries, an HEC of medium MW (2% solution viscosity: 40 cp) is used. The typical fluid-loss-control performance of this material is shown in Fig. 6. A higher-molecular weight HEC is used for lower-density slurries (2% solution viscosity: 180 cp), and the typical performance in bentonite-extended slurries is shown in Fig. 7.
![HEC concentrations required to obtain fluid-loss rates less than 100 mL/30 min with low-density cement slurries. Temperature range: 80 to 150°F [27 to 66°C]. All slurries are API/ISO Class H with 0.5% PNS (BWOC) and fresh water.](https://www.drillingmanual.com/wp-content/uploads/2021/04/image-85.png)
HEC, as well as hydroxypropyl cellulose (HPC), with a DS range of about 0.9 to 2.8 and a MS range of about 1.0 to 6.0, are disclosed as fluid-loss control additives when used with high-molecular-weight xanthan gum (MW 2,000,000)
Cellulosic fluid-loss additives generally share certain disadvantages. They are effective water viscosifiers; as a result, they can increase the difficulty of slurry mixing and ultimately cause undesirable cement-slurry viscosification. At temperatures less than about 150°F [65°C], cellulosic fluid-loss additives are efficient cement retarders; thus, care must be taken to avoid slurry overretardation. Also, as shown in Fig.6, the efficiency of the cellulose polymers decreases with increasing temperature.
Recent changes in environmental regulations around the world have encouraged the development of more environmentally acceptable cement additives. Biopolymers such as celluloses are very attractive because they pose little or no risk to the environment. Therefore, work has been performed to extend the useful range of cellulosic fluid-loss additives.
Mueller and Bray (1993) found that adding ethoxylate-coated resins in concert with HEC (and also PVA) significantly improved fluid-loss performance. Greater efficiency, salt tolerance, and thermal stability were reported.
A low-molecular-weight ethoxylated HEC (MW = 60,000), with 1 to 4 moles of ethylene oxide per anhydroglucose unit, was recently introduced (Dao and Vijn, 2002; Vijn et al., 2002). It is effective at temperatures up to 280°F [138°C] and can be used in cement slurries prepared with fresh water, seawater, or salt-saturated mix water. A temperature-stabilizing agent, such as synthetic hectorite [Na 0.4 Mg 2.7 Li 0.3 Si 4 O 10 (OH) 2 ], sodium thiosulphate, or magnesium oxide, can also be added.
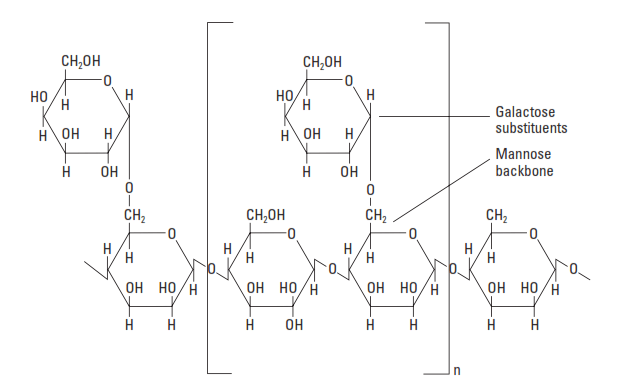
Natural Polymers – Galactomannans
Galactomannans are natural polysaccharides essentially consisting of galactose and mannose units (Fig. 8). They are produced from the endosperm of leguminous seeds such as guar or carob. Guar gum is a polymer that contains monomeric units of D-mannose linked to one another by 1–4 bonds forming the main chain on which units of D-galactose are branched by 1–6 bonds. In the oil field, guar and guar derivatives have been used for many years to viscosify hydraulic fracturing fluids. A hydrophobically modified hydroxy propylated guar (HPG), with a MW of less than 2,000,000, can provide fluid-loss control at temperatures up to about 230°F [110°C] (Audibert et al., 2001).
Synthetic polymers – Nonionic synthetic polymers
Polyvinylpyrrolidone (PVP) may be used with PNS dispersants (Boncan and Gandy, 1986). It is also known to improve fluid-loss control when added with CMHEC (Hale, 1981) or HEC (Chatterji and Brake, 1982; Chatterji, et al., 1984).
Complex mixtures containing polyvinylpyrrolidone, maleic anhydride-N-vinylpyrrolidone copolymer, and poly(aryl-vinylbenzyl) ammonium chloride, i.e., polycations (Wahl and Dever, 1963), have been reported as effective fluid-loss control additives. In addition, N-vinylpyrrolidone can be copolymerized with styrene sulfonate (SS) to form an effective fluid-loss control additive (Newlove et al., 1984; Sedillo et al., 1987).
PVA is frequently used as a fluid-loss control additive (Harrison, 1968; Carpenter, 1986; Moran and Moran, 1998a and 1998b). This material is particularly advantageous for low-temperature applications [less than 100°F (38°C)] because it has no retarding effect and is compatible with cement slurry accelerators such as calcium chloride. The fluid-loss control behavior of PVA is shown in Fig. 9. It is important to note the sharp threshold effect associated with this additive. Within a very short concentration range, the fluid-loss rate falls from 500 mL /30 min to less than 20 mL /30 min. Therefore, to ensure adequate fluid-loss control, special care is necessary during field blending operations to verify the correct additive concentration.
PVA can be crosslinked to form a microgel that is not water-soluble.
![API/ISO fluid-loss rate versus PVA concentration in a
Class A cement slurry with 46% water and 2% calcium chloride at
100°F [38°C] temperature and at 1,000 psi [7 MPa] of pressure.](https://www.drillingmanual.com/wp-content/uploads/2021/04/image-88.png)
Class A cement slurry with 46% water and 2% calcium chloride at
100°F [38°C] temperature and at 1,000 psi [7 MPa] of pressure.
Synthetic polymers – Anionic synthetic polymers
Anionic synthetic polymers The largest group of anionic polymer fluid-loss additives is composed of co- or terpolymers derived from acrylamide (AAm). Polyacrylamides are nonionic and are not used by themselves as fluid-loss additives, although they can provide some fluid-loss control. They are subject to rapid hydrolysis to acrylic acid (AA), resulting in severe cement-slurry retardation (Crema et al., 1989).
Partially hydrolyzed polyacrylamide, containing vari- ous proportions of acrylic acid or acrylate units, is often added to drilling fluids. However, it is difficult to use in well-cement slurries owing to the strong interaction between the carboxylate groups and cement grain surfaces, often resulting in retardation or flocculation. Nevertheless, some applications have been reported using a material with a low AA/AAm ratio, about 0.1 (McKenzie and McElfresh, 1982).
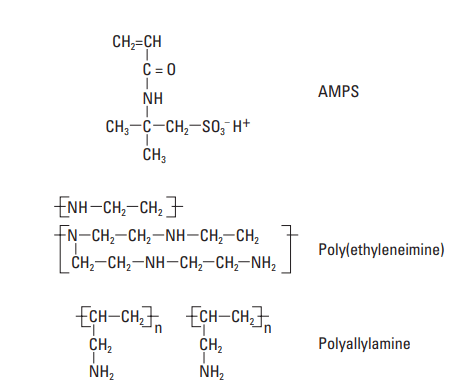
The copolymers of AAm most often described in the patent literature contain a sulfonate monomer: 2-acrylamido-2-methyl propane sulfonic acid (AMPS) (Mueller, 1992). The structural formula is shown in Fig. 10. AMPS has been copolymerized with the following materials to produce fluid-loss control agents.
- AAm (Persinski et al., 1977; Boncan and Gandy, 1986; Oswald et al., 2001; Walker, 2002)
- N,N-dimethylacrylamide (NNDMA) (Rao and Burkhalter, 1986; Brothers, 1987; George and Gerke, 1985; Fry et al., 1987; Chatterji et al., 2001). Terpolymers of AMPS are also used, as listed below.
- AMPS+ AAm + itaconic acid (IA) (Savoly et al., 1987)
- AMPS + AA + N-methyl-N-vinyl acetamide (NMVA) (Defossé, 1985)
- AMPS + vinyl sulfonate + NMVA (Hille et al., 1987)
- AA(AAm) + NMVA + AMPS (Hille et al., 1987)
AMPS may also be part of a copolymer or a terpolymer grafted to a lignin backbone and associated with acrylonitrile, NNDMA, or AA. These complex polymers are efficient in salt slurries (Fry et al., 1987).
A polymer composition comprising the random polymerization product of acryloylmorpholine with at least one and, preferably, two other monomers within the group consisting of AMPS, N-vinyl pyrrolidone, and nylphosphonic acid, is effective to reduce fluid loss of cement slurries at temperatures up to 400°F [204°C] (Udarbe and Hancock-Grossi, 2000).
A tetrapolymer composed of AMPS, N-vinyl-2-pyrrolidone, AAm, and acrylic acid controls fluid loss of cement slurries at temperatures up to 450°F [232°C] (Stephens, 1994). Typical performance of this additive is illustrated in Fig. 11.
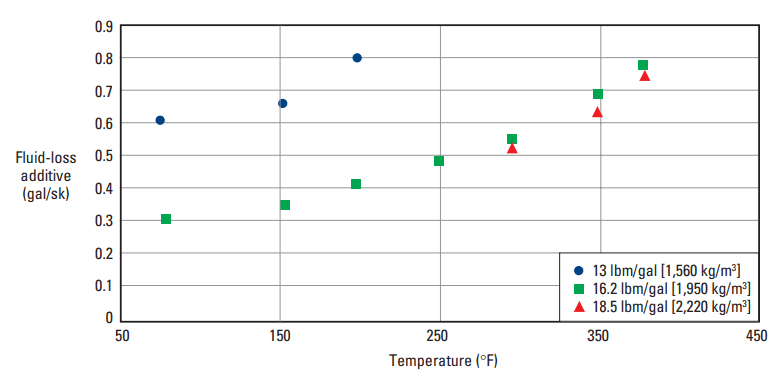
tetrapolymer versus temperature. The plot shows the fluid-loss additive concentration in a Class H cement
necessary to achieve an API/ISO fluid-loss rate of less than 50 mL/30 min.
Copolymers containing AAm, 3-allyloxyhydroxypropanesulfonate, and other monomers are reported to exhibit excellent fluid-loss control properties at temperatures ranging from 80°F [27°C] to 350°F [177°C] (Bair et al., 2002). They are not salt sensitive and can tolerate a salt concentration up to saturation.
A tannin grafted with AMPS and AAm provides fluidloss control at temperatures up to 400°F [204°C] (Huddleston et al., 1992). This polymer is also reported to prevent gas migration through the cement slurry (Eoff and Loughridge, 1994)
Sulfonated polyvinyl aromatics such as sulfonated polystyrene (SPS) (Martin, 1966; Newlove et al., 1984; Sedillo et al., 1987) and sulfonated polyvinyltoluene (SPVT) (Wahl et al., 1963) have been identified as useful fluid-loss control agents. A blend of SPVT, PNS, and a sulfonated copolymer of styrene and maleic anhydride is effective in salt cement systems (Nelson, 1986). The fluid-loss control performance of this material in a saltsaturated cement slurry is shown in Fig. 12.
![Fluid-loss-control performance of a blend of sulfonated polyvinylaromatic polymers in a 16.7-lbm/gal [2,005-kg/m3 ]salt-saturated Class H cement slurry (37% NaCl BWOW and 40%H2O) at 200°F [93°C].](https://www.drillingmanual.com/wp-content/uploads/2021/04/image-93.png)
Synthetic polymers – Cationic synthetic polymers
Polyethylene imine (PEI), shown in Fig. 10, is an example of a polyalkylene polyamine that was widely used as fluid-loss additive (Gibson and Kucera, 1970; Scott et al., 1970; McKenzie, 1984). The MW range within which PEI is effective is 10,000 to 1,000,000. Its structure is likely to be highly branched; therefore, all three types of amine groups (primary, secondary, and tertiary) should be present in the chain.
The dispersant PNS must be present with PEI to obtain significant fluid-loss control. An insoluble association is made between the two polymers to create particles that provide fluid-loss control. As shown in Fig. 13, fluidloss control improves as the MW of the PEI increases.
The principal advantage of PEI as a fluid-loss control agent is its effectiveness at high temperatures. As shown in Table 3, PEI provides excellent fluid-loss control at circulating temperatures as high as 436°F [225°C]. A notable disadvantage of PEI is its tendency to promote slurry sedimentation. Although the sedimentation is preventable, slurry design can be very difficult.
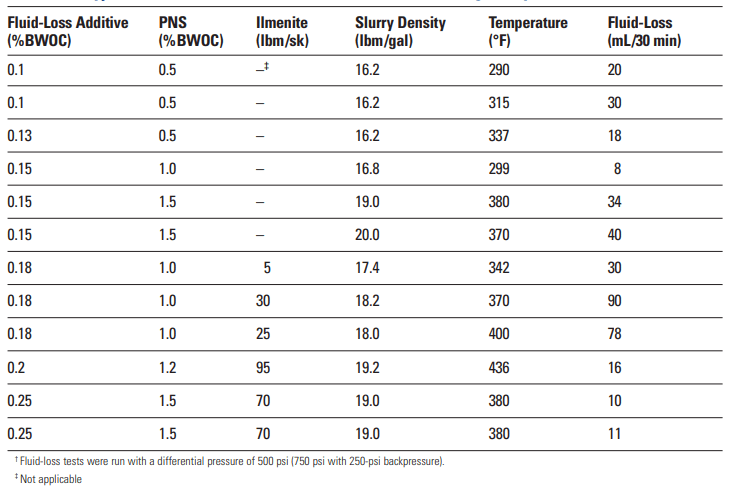
Polyallylamine has been reported by Roark et al. (1986; 1987a, 1987b, and 1987c) as an effective fluid-loss control agent. Instead of being part of the chain backbone, the amine group is a pendant (Table. 4). This material can also be slightly cross-linked to decrease slurry sedimentation. Table 10 shows the fluid-loss control performance of polyallylamine at two MWs.
![Comparison of Two MWs of Polyallylamine Polymers Added at 2% BWOC to 15.8-lbm/gal [1,897 kg/m3] Class G Cement†](https://www.drillingmanual.com/wp-content/uploads/2021/04/image-99.png)
Various quaternary ammonium or sulfonium monomers can be copolymerized with other materials to obtain effective fluid-loss control agents. Several are listed below.
- Alkyl ammonium chloride or sulfonium chloride (Wahl and Dever, 1963)
- Dimethyl-diallyl ammonium chloride (DM-DAAC)
- Methacrylamidopropyltrimethyl ammonium chloride (MAPTAC) (Peiffer et al., 1986; 1987)
The alkylammonium and sulfonium chloride are copolymerized with vinylbenzene to obtain poly(ar-vinyl benzyl) alkylammonium or sulfonium chlorides. DMDAAC is copolymerized with AA or methacrylic acid. It is also copolymerized with AMPS and vinyl phosphonic acid or vinyl phosphonic salts. MAPTAC is copolymerized with SS or AAm. Such materials are ampholytic polymers bearing negative and positive charges at a high pH (such as the aqueous phase of a Portland cement slurry).
Ref: Schlumberger Well Cementing, Erik B. Nelson and Dominique Guillot