Many inorganic salts are accelerators of Portland cement Slurry. Chloride salts are used most frequently. Other salts that have an accelerating effect include carbonates, silicates (especially sodium silicate), aluminates, nitrates, sulfates, thiosulfates, and alkaline bases [e.g., sodium hydroxide (NaOH), potassium hydroxide (KOH), and ammonium hydroxide (NH4OH)].
Ca2+ > Mg2+ > Li+ > Na+ > H2O
OH– > Cl– > Br– > NO3 –> SO4 -2 = H2O
Cement Slurry Accelerators Examples
- Well Blowout
- Jar Intensifier
- Landing Nipple In Well Completion
- Well Control On Rigs: 15 – Checks & Tests
- Snubbing Units In Oil & Gas Field
Calcium Chloride
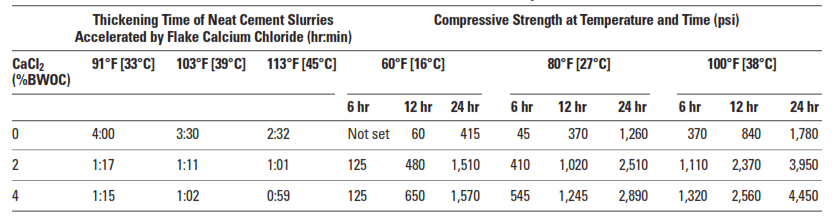
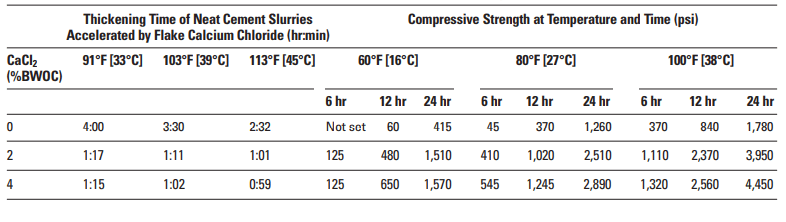
Calcium chloride (CaCl2) is undoubtedly the most efficient and economical of all cementing additives accelerators. Regardless of concentration, it always acts as an accelerator (Table 1). It is normally added at concentrations between 2% to 4% by weight of cement (BWOC). Results are unpredictable at concentrations exceeding 6% BWOC, and premature settings may occur.
Cement Slurry Accelerators Sodium Chloride
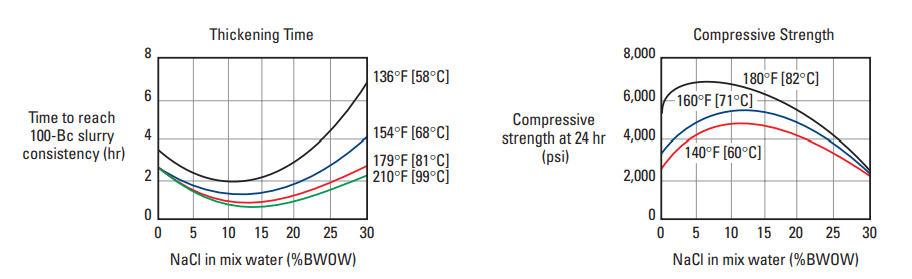
Sodium chloride affects the oil well cement properties thickening time and compressive strength development of Portland cement in different ways, depending upon its concentration and the curing temperature (Fig.1). NaCl acts as an accelerator at concentrations up to 15% by weight of mix water (BWOW). Between 15% and 20% BWOW, NaCl is essentially neutral, and thickening times are similar to those obtained with fresh water. The addition of NaCl at concentrations above 20% BWOW causes retardation. NaCl is not a very efficient accelerator and should be used only when CaCl2 is not available at the well site.
Seawater
Seawater is used extensively for mixing cement slurries at offshore locations. It contains up to 2.5 wt% NaCl, resulting in acceleration. The presence of magnesium in seawater (about 0.15 wt%) must also be taken into account.
Cement Slurry Accelerators – Chloride-free Accelerators
Chloride-free accelerators, originally developed by the concrete industry to reduce the corrosion of reinforcing steel, are also used in well cement. Sodium silicate is normally used as a cement extender; however, it also has an accelerating effect. Sodium silicate reacts with Ca2+ ions in the aqueous phase of the cement slurry to form additional calcium silicate hydrate (C-S-H) phase nuclei, thus hastening the end of the induction period. Other examples include alkaline earth formates, nitrates, nitrites, triethanolamine, and thiocyanates (Pauri et al., 1986; Ramachandran, 1973; 1976a).
Cementing Accelerators Calcium Chloride—Mechanisms Of Action
Calcium chloride is by far the most common accelerator of Portland cement Slurry. The mechanisms by which it operates are complex and still not completely understood. Several hypotheses described in the literature are summarized below.
Effects of calcium chloride on the hydration of principal Portland cement phases
It has been proposed that calcium chloride increases the hydration rate of the aluminate phases–gypsum system (Bensted, 1978; Traetteberg and Grattan-Bellew, 1975). Chloride ions enhance the formation of ettringite until the gypsum is consumed (Tenoutasse, 1978). If free C3A remains, calcium monochloroaluminate (C3A • CaCl2 • 10H2O) forms. The more rapid setting of the cement slurry is also attributed to the crystalline shape of ettringite, which occurs as very fine needles (Bensted, 1978; Young et al., 1973).
By contrast, Stein (1961a) and Edwards and Angstadt (1966) concluded that accelerators do not promote the hydration of C3A but instead accelerate the hydration of C3S. This accelerating action of calcium chloride has been confirmed by studying the hydration of pure C3S (Odler and Skalny, 1971a) and C2S (Collepardi and Massidda, 1973).
Change in C-S-H structure
Many researchers have proposed that Portland cement hydration is controlled by the diffusion of water and ionic species through the initial protective C-S-H phase coating. Therefore, the hydration rate should be influenced by the permeability of the coating. A morphological change of the C-S-H phase to a more open, flocculated structure would enhance diffusion and accelerate hydration. Such a process has been confirmed in studies of pure C3S (Odler and Skalny, 1971a; Ben-Dor and Perez, 1976; Traetteberg et al., 1974). The C-S-H phase has a higher bulk lime-to-silica (C/S) ratio and a “crumpled-foil” morphology rather than a spicular or needle-like appearance. In the presence of calcium chloride, C-S-H phase has a greater specific surface area (Collepardi and Marchese, 1972) and a greater degree of silicate-anion polymerization (Hirljac et al., 1983). A change in the pore-size distribution of hydrated C3S (Young et al., 1973; Skalny et al., 1971) and C2S (Odler and Skalny, 1971b) has also been reported. The morphology of calcium hydroxide (portlandite) is also affected by the presence of chloride ions (Berger and McGregor, 1972). The portlandite crystals become elongated.
Diffusion of Accelerators chloride ions in Cement Slurry
Kondo et al. (1977) determined the diffusion rate of anions and cations from alkaline and alkaline-earth chlorides through a set–Portland cement plate. They concluded that the diffusion coefficient of the chloride ion is much higher than that of the accompanying cation. Because the chloride ions diffuse into the C-S-H phase layer more quickly than the cations, a counterdiffusion of hydroxyl ions must occur to maintain the electrical balance. Therefore, the precipitation of portlandite, ending the induction period, takes place earlier. Kondo et al. (1977) also established that only a small amount of chloride is incorporated into the C-S-H lattice but that it may be chemically bound to the C-S-H surface.
Others studied the effect of calcium chloride on C3S hydration. They concluded that the accelerating effect occurred because chloride ions have a smaller ionic size than hydroxyl ions and would more easily diffuse into the C-S-H membrane. The resulting internal-pressure increase takes place more quickly, causing the C-S-H membrane to burst earlier.
Change in aqueous phase composition In Cement Slurry
Michaux et al. (1989a and 1989b) showed that calcium chloride strongly modifies the distribution of ionic species in the aqueous phase of well-cement slurries. Chloride ions do not participate in the formation of hydration products during the induction period; therefore, one observes a decreased concentration of hydroxyl and sulfate ions and an increased concentration of calcium ions. Kurczyk and Schwiete (1960) proposed that calcium chloride decreases the alkalinity of the aqueous phase, resulting in acceleration.
Stadelmann and Wieker (1985) investigated the influence of a large number of inorganic salts on the hydration of C3S. They showed that C3S hydration is accelerated by increasing the solubility of calcium hydroxide in the aqueous phase, e.g., by adding CaCl2. Conversely, retardation is observed when the solubility of calcium hydroxide decreases, e.g., by adding high concentrations of NaCl.
Wu and Young (1984) showed that the addition of calcium salts affects the dissolution rate of C3S. When the concentration of calcium in the aqueous phase is monitored with time, the peak concentration always occurs earlier in the presence of chloride ions. Thus, precipitation of calcium hydroxide (and the end of the induction period) occurs earlier.
In conclusion, it is apparent that many factors interact simultaneously in the acceleration of Portland cement by calcium chloride. Both physical and chemical phenomena are involved. The presence of chloride ions alters the structure and increases the permeability of the C-S-H phase layer. In addition, calcium chloride significantly alters the distribution of ionic species in the aqueous phase, resulting in a faster hydration rate.
Secondary Effects Of Cement Slurry Accelerators Calcium Chloride
In addition to the acceleration of the initial set, several other effects are evident when calcium chloride is present in a Portland cement system. Some effects are not beneficial; therefore, calcium chloride should be used judiciously depending upon well conditions. A summary of the more important secondary effects is given below.
Heat of hydration
The presence of CaCl2 increases the rate of heat generation during the first hours after slurry mixing. If the wellbore is thermally insulated to a sufficient degree, the temperature of the cement, casing, and surrounding formation can increase by as much as 50°–60°F [27°– 33°C] after slurry placement. An autoacceleration of hydration results
More importantly, increased casing expansion occurs because of the temperature rise. Because steel casing and cement do not have the same coefficient of thermal expansion, the casing may shrink away from the cement when the hydration heat eventually dissipates. This results in a so-called “thermal microannulus,” and zonal isolation is compromised (Pilkington, 1988). Additional research is needed to better quantify this effect and to determine the most susceptible wellbore environments.
Slurry rheology
According to Collepardi (1971), calcium chloride increases the yield point (check also Yield Point In Drilling Mud Formula) of a cement slurry but initially does not affect the plastic viscosity. After a 30-min hydration period at ambient conditions, the plastic viscosity begins to increase. Slurries containing calcium chloride also tend to have a greater degree of thixotropy; as a result, particle sedimentation is seldom a problem.
Compressive strength development
Calcium chloride significantly increases the rate of compressive strength development during the first few days after slurry placement. The magnitude of this effect depends upon the curing temperature and the CaCl2 concentration (Table 2).
Shrinkage
Calcium chloride has been shown to increase volumetric shrinkage by 10% to 50% in concretes (Shideler, 1952). This is mainly owing to the greater degree of hydration and changes in hydration products (Collepardi and Massidda, 1973). Such data cannot be directly translated to well cements, because the service conditions are very different. To the authors’ knowledge, a thorough investigation of the dimensional stability of calcium chlorideaccelerated well cements has not been performed. The magnitude of the shrinkage effect in concrete and the popularity of calcium chloride as an accelerator for well cement slurry suggest that such a study is overdue.
Permeability
Initially, the permeability of set cement containing calcium chloride is reduced. This is caused by the greater volume of hydration products compared to additive-free cement. Later, when the degree of hydration is similar for both systems, the permeability of the set cement containing CaCl2 is greater than that of its additive-free counterpart (Gouda et al., 1973).
Sulfate resistance
Because the ultimate permeability of calcium chlorideaccelerated systems is greater, the resistance to aggressive sulfate solutions is reduced (Shideler, 1952; Gouda et al., 1973). However, the C3A content of the cement is the principal controlling factor governing sulfate resistance.
Chloride-free Cementing Accelerators
Although calcium chloride is the least expensive and most effective accelerator, growing concern about its corrosion of the reinforcing steel embedded in Portland cement concrete slurry has led to the development of chloride-free additives. Casing corrosion from chloride accelerators may also be a concern during the life of a well.
Under normal conditions, steel reinforcement is protected from corrosion (passivated) by the high pH of the surrounding concrete-pore solution. A thin protective film of gamma ferric oxide (γ-Fe2O3) forms on the steel surface. The protective film prevents iron cations (Fe2+) from entering the electrolyte and acts as a barrier to prevent oxygen anions (O2–) from contacting the steel surface. If the passivation is compromised, corrosion of the reinforcement can occur at a high rate. The protective film can be disrupted by a significant reduction of the pore-solution pH because of carbonation or by the penetration of aggressive ions such as chlorides to the steelconcrete interface.
A plethora of inorganic and organic compounds, including alkali carbonates, alkali silicates, alkali aluminates, alkali sulfates, alkali hydroxides, nitrates, nitrites, thiocyanates, thiosulfates, formates, and alkanolamines, have been evaluated as calcium-chloride replacements. However, very few have performed well enough to be used on an industrial scale. Unlike calcium chloride, which is generally added alone, most of the commercial chloride-free accelerators are formulated and contain several components.
Calcium formate, Ca(HCOO)2, was patented as a cement slurry accelerator in 1965. Owing to its low solubility in water, calcium formate is usually sold as a powder. The normal dosage is 1–2% BWOC. Calcium formate accelerates the hydration and setting of all types of Portland cement, but its effect is not significant within the first 24 hr. Early strength development can be improved by including sodium nitrite as an accelerator aid (Rosskopf et al., 1975).
The use of calcium nitrite, Ca(NO2)2, as an accelerator was patented by Angstadt and Hurley in 1963. Its water solubility is very high; therefore, it can be used as a liquid additive. The effect of calcium nitrite on strength development is comparable to that of calcium chloride (Rosenburg et al., 1977). Calcium nitrite is also an effective corrosion inhibitor for steel embedded in concrete (Berke, 1985).
Calcium nitrate, Ca(NO3)2, in conjunction with triethanolamine, is an effective accelerator (Tokay, 1982); however, its efficiency is highly variable depending on the cement type. Technical calcium nitrate, a blend of calcium- and ammonium-nitrate hydrates, has a dual function as a set accelerator and corrosion inhibitor for reinforced concrete (Justnes and Nygaard, 1995).
Thiocyanate (SCN–) salts were introduced to the concrete market in 1983 (Rosskopf, 1983). Like the nitrates, the thiocyanates should be accompanied by an alkanolamine to attain the desired results. The combination of thiocyanate salts with nitrates and alkanolamines was patented in 1984 (Gerber, 1984).
Triethanolamine, N(C2H4OH)3, accelerates the reaction between C3A and gypsum and, at dosages of 0.1 and 0.5% BWOC, setting can occur within a few minutes at ambient temperature (Ramachandran, 1973; 1976b). However, compressive strength development can be delayed because triethanolamine strongly retards C3S and C2S hydration. Therefore, triethanolamine is rarely used by itself as an accelerator.
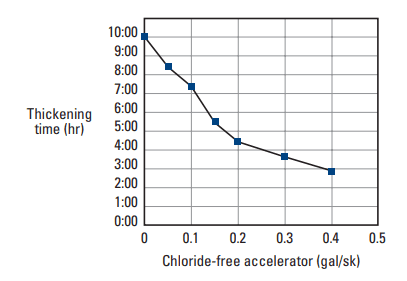
A complex mixture of calcium nitrate, calcium nitrite, diethylene glycol, methyldiethanolamine, and calcium bromide was recently patented as an accelerator for well cement slurry (Maberry et al., 2001). It is used in cold cementing environments such as deepwater offshore wells or permafrost zones. The performance of this additive (Fig. 2) is superior to calcium chloride at temperatures between about 40° and 70°F [5° and 20°C]. Unlike calcium chloride, this accelerator does not affect slurry rheology at elevated concentrations.
Ref: Schlumberger Well Cementing, Erik B. Nelson and Dominique Guillot