Monitoring and controlling the potassium ion concentration for water based mud systems that use potassium for shale inhibition is important. It will deplete as it is adsorbed onto shales. Depletion must be monitored, and effective inhibition levels must be maintained in the mud system. The Potassium ion concentration is determined by adding excess Sodium Perchlorate to a measured mud filtrate volume to precipitate out Potassium Perchlorate. The precipitate is then centrifuged, and the volume of the compacted precipitate is converted to Potassium ion concentration by reference to a calibration curve, which must be prepared using the test reagents at the rig site (see overleaf).
Equipment Required For Measuring Potassium Ion Concentration
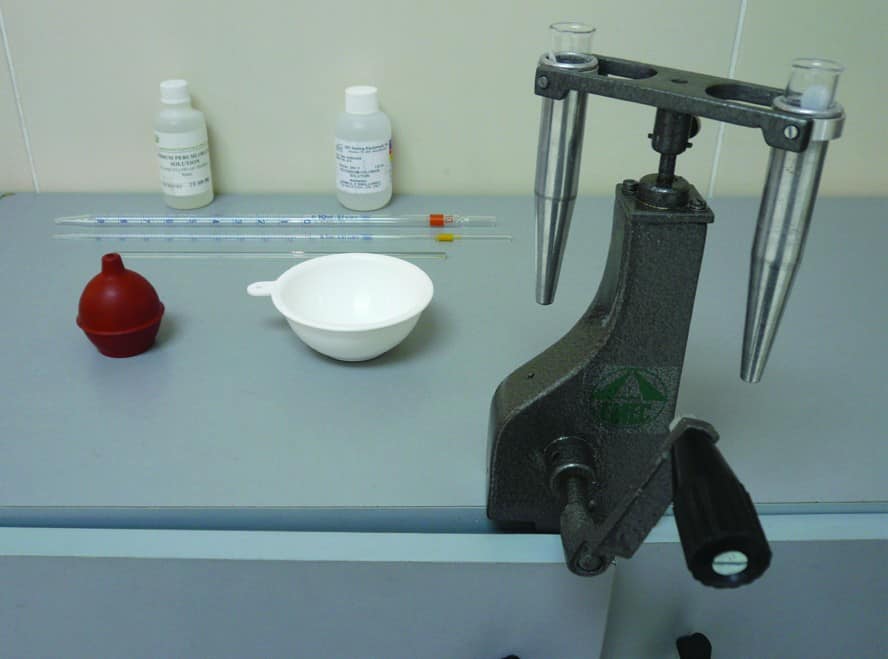
The following equipment is required for measuring Potassium ion concentration:
- Standard Sodium Perchlorate solution (150.0 g per 100 cm3 de-ionized water)
- Standard Potassium Chloride solution (14.0 g per 100 cm3 de-ionized water)
- Hand-crank or electrical centrifuge (1,800 rpm)
- Graduated Pipettes (1 ml and 10 ml)
- Graduated centrifuge tubes (10 ml)
- Calibration curve for Potassium Chloride
Calibration Curve
A calibration curve must be prepared using the test reagents at the rig site. The calibration curve will most likely be similar to those prepared on previous wells. Still, a new curve must always be prepared if there are variations in reagent concentrations.
The calibration curve is prepared as follows:
- Prepare Standard Potassium Chloride solution using 14.0 gm Potassium Chloride powder made up to 100.0 cm with de-ionized water.
- Use a pipette to transfer 0.5 ml Standard Potassium Chloride solution to a clean, empty graduated centrifuge tube, dilute to the 7.0 ml mark with de-ionized water and agitate. This gives 3.5 ppb KCl concentration, but higher KCl concentrations can be prepared in the centrifuge tube for all the other points in the calibration curve, as shown in Fig.1 below.
- Add 3.0 ml Sodium Perchlorate solution to the graduated centrifuge tube but do not agitate – a white precipitate is formed.
- Place the centrifuge tube in the centrifuge and counterbalance the centrifuge with a similar centrifuge tube filled with water. Centrifuge at a constant speed (around 1,800 rpm) for one minute and read the volume of the precipitate immediately afterward. The speed of an electrical centrifuge can usually be adjusted to 1,800 rpm using the dial. The speed of a hand-crank centrifuge can be adjusted to 1,800 rpm by turning the crank handle slowly and counting the number of centrifuge arm revolutions for one full turn of the crank (handle). The number of crank turns required to obtain 1,800 rotor revolutions can be calculated from this. Dividing the number of crank turns required for 1,800 revolutions by 12 will give the number of handle turns required every 5 seconds, and the hand crank speed rate can then be adjusted to obtain 1,800 rpm by using a stopwatch. For example, if one full turn of the hand-crank centrifuge handle produces ten full centrifuge arm revolutions, then 180 handle turns are required to obtain 1,800 centrifuge arm revolutions. Therefore, a centrifuge speed of 1,800 rpm can be obtained by 180 full handle turns per minute, equivalent to 3 full handle turns per second or 15 full handle turns every 5 seconds.
- Repeat Steps 2 to 4 using higher KCl concentrations as tabulated above. Always use a pipette when transferring the required amount of Standard Potassium Chloride solution to the clean, empty graduated centrifuge tube. Plot the volume of Precipitate (ml) against KCl Concentration (ppb), from which a straight line can be produced, similar to that shown below.
| Standard KCl Solution | De-Ionized Water | Final Volume | KCl Concentration |
| 0.5 ml | 6.5 ml | 7.0 ml | 3.5 ppb |
| 1.0 ml | 6.0 ml | 7.0 ml | 7.0 ppb |
| 1.5 ml | 5.5 ml | 7.0 ml | 10.5 ppb |
| 2.0 ml | 5.0 ml | 7.0 ml | 14.0 ppb |
| 2.5 ml | 4.5 ml | 7.0 ml | 17.5 ppb |
| 3.0 ml | 4.0 ml | 7.0 ml | 21.0 ppb |
| 3.5 ml | 3.5 ml | 7.0 ml | 24.5 ppb |
| 4.0 ml | 3.0 ml | 7.0 ml | 28.0 ppb |
| 4.5 ml | 2.5 ml | 7.0 ml | 31.5 ppb |
| 5.0 ml | 2.0 ml | 7.0 ml | 35.0 ppb |
| 5.5 ml | 1.5 ml | 7.0 ml | 38.5 ppb |
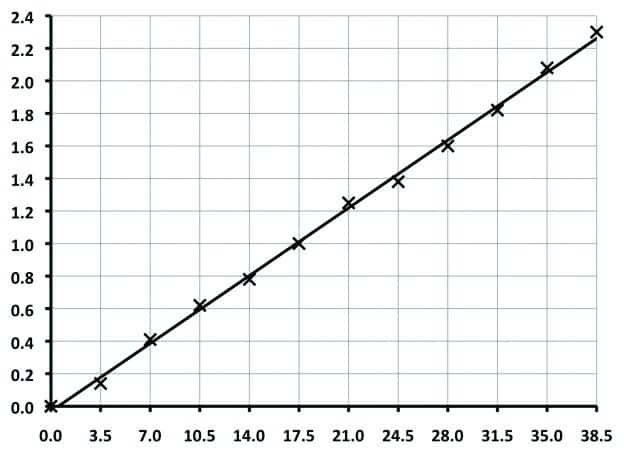
KCl Concentration Measurements
The concentration of KCl in the mud can be measured once the calibration curve has been prepared, as follows:
- Use a pipette to transfer 7.0 ml mud filtrate into the graduated centrifuge tube.
- Add 3.0 ml Sodium Perchlorate solution, but do not agitate. If potassium is present, then a white precipitate is formed at once.
- Counterbalance the centrifuge with a similar centrifuge tube filled with water, centrifuge at 1,800 rpm for one minute, and read the volume
of the white precipitate. - Use the calibration curve to convert the precipitate volume to KCl concentration.
- Add 2 or 3 drops of Sodium Perchlorate to the centrifuge tube after centrifuging to confirm that all the Potassium has been precipitated out
by the Sodium Perchlorate. If more precipitate appears, then the KCl concentration is too high for the test procedure, and the test should be
repeated using a smaller mud filtrate volume as per the following table:
| KCl (lb/bbl) | K+ (mg/l) | Filtrate Volume (ml) |
| 3.5 to 18 | 5,250 to 27,000 | 7.0 |
| 18 to 35 | 27,000 to 52,500 | 3.5 |
| 35 to 70 | 52,500 to 105,000 | 2.0 |
| Above 70 | Above 105,000 | 1.0 |
The smaller filtrate volume should then be increased to 7 ml with de-ionized water and agitated before adding the Sodium Perchlorate. When smaller filtrate volumes are used, the KCl concentration of the mud system can be calculated using the opposite Eq.1 below.
The KCl depletion rate will depend on the shale reactivity and drilling ROPs, so regular KCl treatments may be required to maintain concentration at the required level for effective inhibition. The Potassium concentration can be converted to other units as shown in Eq.2 below.
Calculation for determining Potassium Chloride Concentration.
Eq.1 : KCI Concentration (ppb) = ( 7 / Filtrate Volume (ml) )x KCI Concentration from Calibration curve
Conversion factors for reporting Potassium Chloride Concentrations in different units.
Eq.2: KCl (gm/l) = KCl (ppb) x 2.85714
KCl (% Vol) = KCl (ppb) ÷ 3.5
K + = KCl (ppb) x 1,500
very educative. I need lectures on drilling fluids and job adverts on mud engineering.